XI. SYNTHETIC LUBRICATING OIL MANUFACTURE - LEUNA
Information on this subject was secured at Leuna by inspection of the plant and by interviews with the following I.G. personnel (May 8-15, 1945):
Dr. Giessen - in charge of organic chemicals manufacture.
Dr. Gericke - in charge of operation of the lubricating oil plant and some smaller chemical plants.
Dr. Zorn - in charge of lubricating oil research for the past 15 years
SUMMARY OF PROCESS:
Ethylene of 95% purity is obtained by thermal cracking of ethane in admixture with oxygen. The ethylene is polymerized batchwise in the presence of a special aluminium chloride catalyst. The polymerized oil is separated from the aluminium chloride complex, steam distilled to remove light ends, and given a final clay treatment before use.
The plant was designed for the production of 200 B/CD (850 metric tons/month) finished lubricating oil from 1630 MCF/D (60°F - 760 mm.) of ethane. Actual production in continuous operation was about 85% of this figure. Yield of finished lube oil from original ethane was about 48.5 weight per cent.
PROCESS DETAILS:
The overall material balance for the plant is given in Fig. XXV based on 170 B/CD (700 T/Mo) lubricating oil production. details of processes are given in Figs. XXVI, XXVII, XXVIII, XXIX, based on design stream day operation where available.
1) Thermal Cracking of Ethane
The ethane cracking equipment consists of four parallel units, each with an ethane charge capacity of 617 MCF/D. Ethane (three volumes) and oxygen (one volume) are preheated separately at atmospheric pressure in gas-fired tubular heaters to 1100-1200°F and 750°F, respectively. The heaters are constructed of Sicromal (8-12% Cr). The hot gases are mixed during passage through a refractory assembly (“tulip”) in the bottom of the subsequent ethane cracking furnace. The mixture passes upward through the cracking chamber, which is filled with 1-1¼” dia. ceramic spheres. Cracking conditions are approximately 1616° F and 300-400 mm. mercury absolute pressure. The cracked gases are quenched by a water cooled heat exchanger and then by direct injection of water. Vacuum is held on the cracking furnace by a water sealed vacuum pump which discharges the cooled gases to the next stage of the process.
It is interesting to note that the design capacity of four cracking furnaces is 2470 MCF/D ethane, producing 4,200 MCF/D water-free cracked gas; actual requirement (once through) for the 200 B/D lubricating oil production is 1,825 MCF/D, indicating a 75% stream time efficiency. The factor determining stream time efficiency is coking of the lower section of the ethane cracking furnace where oxidation occurs. A small amount of nitrogen (up to 2% of the ethane) is added to the ethane cracking furnace as a control of cracking.
The products of cracking are next washed with caustic soda in order to remove organic acids and formaldehyde.
2) Acetylene removal:
Acetylene is removed by hydrogenation over a nickel-chromium catalyst at a temperature of about 400°F, atmospheric pressure and a gas space velocity of 1,165 volumes gas (at standard condition)/volume catalyst/hour. Linear velocity was stated to be 0.33-0.39 ft/sec. but vessel dimensions indicated a greater linear velocity at the above space velocity. The hydrogen required for hydrogenation is already present in the cracked gas. Any oxygen present in this gas is hydrogenated to water.
After hydrogenation, the four gas streams are combined in one gas holder. From this point the gas is compressed to 220-264 psig. and undergoes four further purification processes at this pressure.
3) Oil Absorption:
The gas is passed counter-current to a middle oil (from coal hydrogenation) for absorption of benzene and high hydrocarbons. The rich oil is stripped with stem in the usual way.
4) Activated Charcoal Absorption:
The gas is next passed through towers containing activated charcoal (rounded cubical pellets 13/64” diameter from Carbo-Norit Union, Leverkusen) in order to remove the last traces of benzene. These towers are operated on a 24-hour absorption/24-hour regeneration cycle. Regeneration is accomplished by steaming at 374°F followed by drying and cooling with nitrogen. The effectiveness of the absorption is checked by cooling a gas sample down to -76°F and noting whether there is any condensation.
5) Alkazid Washer:
The bulk of the CO2 is removed by counter-current washing with an alkazid solution (“m-lauge”, α-amino propionic acid). The alkazid solution is regenerated by heating and steam stripping at atmospheric pressure. The exit gas contains 0.1 to 0.5% carbon dioxide.
6) Caustic Washer:
Four counter-current towers in series are used, each circulating individually. The last tower in the series is filled with new 10% caustic soda (15° Be), and the caustic solution is transferred stepwise to the preceding tower in the system. The caustic in the first tower is dumped when 90% of the available sodium hydroxide has been converted to the bicarbonate. The caustic soda is not regenerated.
To ensure complete removal of carbon dioxide, a sample of exit gas is continuously bubbled through a barium hydroxide solution.]
7) Linde Low Temperature Fractionation:
This plant was designed by the Linde Company for the separation of the washed gas into four fractions. The gas is dried over silica gel, precooled and fed into the first tower under pressure. The first tower produces methane and permanent gases as overhead product and ethylene and heavier as bottoms product. Reflux is secured by a methane compression refrigeration cycle. The overhead product is put to fuel gas.
The bottoms from the first tower expand into the second tower at about 7 psig. This tower yields ethylene and ethane overhead and propylene and heavier hydrocarbons are passed from the kettle to fuel gas. Reflux is provided by expanding ethylene directly into the top of the tower.
The ethylene + ethane overhead fraction passes to the third tower, which gives ethylene as an overhead product and ethane as a bottoms product. Reflux is provide in the same manner as in the second tower. The ethylene is compressed 1200-1500 psi for storage and the ethane fraction is recycled to the ethane cracking furnace. This recycle constitutes about 26.5% of the total feed to the furnace.
Reboil heat is supplied indirectly to all three towers by relatively warm ethylene from the discharge of the ethylene refrigeration compressors. The general heat exchange arrangement is shewn in the attached flow sheet, Fig. XXVIII.
The three towers are copper lined and all equipment inside the towers is made of copper. The three towers and part of the heat exchanger system are enclosed in an insulated box. Two parallel plants are used for this fractionation at full load.
8) Polymerization of Ethylene to Lubricating Oil:
The reaction is carried out in batch autoclaves. The Leuna plant has ten reactors, four of 400 cu.ft. capacity each and six of 290 cu.ft. capacity each. A batch can be run through a reactor in 12-14 hours.
The procedure for polymerizing a batch of ethylene comprises the following steps:
The above procedure refers to the preparation of an oil to a final viscosity at 220 S.S.U. at 210°F (6° Engler at 100°C). to produce an oil of viscosity 105 S.S.U. at 210°F. (3° Engler at 100°C), the polymerization temperature is controlled at 356°F. The yield of oil from ethylene is reduced at the higher temperature..
The first Leuna autoclaves were made from steel containing 12-16% Cr and the oil manufactured was of the desired high viscosity. Subsequently more reactors were added, and these were manufactured from ordinary carbon low viscosity oil (3-4° Engler) for 3 to 4 months, but subsequently gave a high viscosity oil. The plant at Schkopau (see following section), operating on a higher-purity ethylene, had no difficulty making a high viscosity oil in carbon steel reactors from the first. Gericke believes that from this and other evidence, a high-purity ethylene is essential and the impurities in the ethylene, as well as reactor wall material, may influence polymerization. No corrosion difficulties have been experienced at Leuna as long as the equipment is kept absolutely dry.
9) Catalyst Separation and Disposal:
The contents of a reactor are pressured into a batch separation vessel. If the oil and catalyst complex layers do not readily separate, a small amount of methanol is added. The total mixture then passes through a scraper-type (schäl) centrifuge, in which the heavier complex is continuously scraped from the outer drum and retained for further processing. The oil may then pass through a DeLaval centrifuge for further catalyst separation. The optimum temperature for catalyst separation is about 194°F.
The oil, still containing a small amount of complex, next goes into an autoclave in which methanol (2% on oil) and lime dust are added. This treatment breaks down the catalyst complex and neutralizes the acid formed. The entire contents of this autoclave are put through a filter press and the filtered oil is stored prior to distillation.
AlCl3 complex recovered by centrifuging is decomposed in batches by the addition of water. Any oil separating in this process can be either treated and fractionated with the main oil stream or recovered separately.
The filter cake from the filter presses can be extracted with the light oil fraction from the process for additional heavy oil recovery, either by washing in situ or subsequently in batch autoclaves. The total extract oil can be recombined with the main oil stream before the filter presses.
The gas liberated from the purging and venting of the polymerization autoclaves and from the centrifuges and adjacent receivers is water washed, caustic washed and its ethylene content recovered.
All vessels using methanol are provided with methanol reflux systems, after which the gases still vented are water washed before being discarded. The wash water is saved for methanol recovery.
10) Oil Fractionation:
The combined oil from the filter presses is steam distilled at atmospheric pressure to remove about fifteen per cent light ends. These light oils are used in making up catalyst slurry in the polymerization reactors.
The product from the base of the still is batch treated with about 2.5% of a natural Fuller’s earth (from near Munich) at 158°F., filtered through presses to give the finished oil.
In some cases lime and silica gel are used in the final treatment instead of Fuller’s earth.
OPERATING REQUIREMENTS
Utilities: The following utility requirements for the Leuna plant were given, based on one barrel of finished lubricating:
|
Ethane Cracking & |
Polymerization |
||
|
H.P. Steam (220psi), pounds |
1080 |
430 |
|
|
L.P. Steam (22 psi) pounds |
650 |
1950 |
|
|
Water (from River- Once-through) |
|||
|
Winter (40-50°F), gallons |
11200 |
3700 |
|
|
Summer (86°F), gallons |
22400 |
3700 |
|
|
Nitrogen, Cubic Feet |
1,350 |
||
|
Electricity, KWH |
450 |
160 |
|
|
Fuel Gas (250 BTU/CF, lower heating value), BTU. |
1.51 x 106 |
0.10 x 106 |
|
Operators: The following operating personnel was required for the plant. Those figures are based on a 56-hour week, or 3 men per job for 24-hour operation. They do not include any maintenance labour.
|
Total |
Per Shift |
|||
|
Operators |
Foremen |
Operators |
Foremen |
|
|
Ethane Cracking |
18 |
3 |
6 |
1 |
|
Ethylene Purification |
87 |
3 |
29 |
1 |
|
Polymerization & catalyst separation |
68) |
6 |
23) |
2 |
|
Distillation |
12) |
4) |
||
|
Final refining of lubricating oil |
10 |
10 (Days Only) |
- | |
|
General Plant Foreman (Gericke) |
1 |
1 (Days Only) |
||
|
Laboratory |
7 |
1 |
2 |
1 |
|
Total: |
Day Shift: 74 |
6 |
||
|
202 |
14 |
|||
|
Night Shift: 64 |
4 |
|||
The total of 105 operators in the ethane cracking and purification plant might be reduced to 80 if more skilled operators were available.
Normally the plant is shut down for inspection for a 3-day period every 60-120 days. to come on stream from a complete shut-down requires about two weeks.
TESTS ON PRODUCTS
As indicated above, the plant could be operated to produce either a 6° or a 3° Emgler viscosity oil (at 100° C). These products from Leuna were referred to as SS (Schmierstoff) 906 and 903 respectively and specifications for the products were as follows:
|
SS.906 |
SS.903 |
|
|
Specific Gravity at 20°C Max |
0.862 |
0.860 |
|
(API at 60°F. Min.) |
31.9 |
32.3 |
|
Viscosity at 50° C (Engler) |
44.46 |
14-15 |
|
Viscosity at 100° C (Engler) |
5.63 (Min) |
3.0 (Max) |
|
(SSU at 130° F) |
1200-1250 |
400-440 |
|
(SSU at 210° F) |
205 (Min) |
105 (Max) |
|
Viscosity Index. Min. |
107 |
115 |
|
Slope constant “M” in Pole Height Calc., Max. |
3.05 |
3.20 |
|
Pole Height Max. |
1.73 |
1.60 |
|
Pour Point °F, Max. |
-13 |
-31 |
|
Flash Point, °F, max. |
435 |
392 |
|
Neutralization No. (mg KOH/g) |
0.06 |
- |
|
Saponification No., Max. |
0.30 |
- |
|
Noack Evaporation Test (482° F) Max. % |
8 |
- |
|
Conradson Carbon Residue, Max. |
0.2 |
0.2 |
|
Ash, Asphalt, Water |
0 |
- |
The SS 906 product was usually blended with an equal quantity of bright stock derived from petroleum oil, the blend being used as an aviation lubricating oil (denoted S-3).
The natural lubricating oils used in the blend were from three sources, all from refining Austrian crudes, and were denoted as follows:
607 - From Renania, Harburg. Vacuum distilled oil, refined with sulphuric
acid.
707 - From Vacuun Oil Co., near Bremen. Duo-Sol refined.
807 - From Nerag, near Hanover, Furfural refined.
Alternate stocks to the SS 906 (Leuna) product in this S-3 blend were the following:
SS 1106 - 6° Engler (100°C) oil by polymerization at Pölitz of olefins
obtained by cracking paraffin.
SS 1006 - 6° Engler (100°C) oil by polymerization at Rhenania, Harburg, of
olefins obtained by cracking paraffin.
SS 906 - 6° Engler (100°C) oil by polymerization at Schkopau of ethylene
produced, via acetylene, from calcium carbide.
The specification for the S-3 blended aviation lubricating oil is given below:
| Viscosity, Min. 2.8 Engler at 100°C | (98 SSU at 210°F) |
| Viscosity Index Min. | 95 |
| Flash, F., Minimum | 428 |
| Pour Point, Maximum | -4 |
| Conradson Carbon Residue, Max. | 0.3 |
A 3° Engler (100°C) oil from Pölitz (SS 1103 was sometimes used directly Maximum production of this grade was 200 Te/yr (4 B/D).
A standard engine test was used for the determination of the ring-sticking tendencies of aviation lubricating oils. As a reference oil, a refined petroleum lubricant (Vacuum Oil Co. “Rotring”) was used, and results were reported in terms of hours operation before ring-sticking. The various oils described above compared as follows:
|
Reference oil (Rotring) |
8 hours |
|
Synthetic oil (Leuna)100 SSU/210 (SS 903) |
32 hours |
|
Synthetic oil (Pölitz)100 SSU/210 (SS 1103) |
20-22 hours |
|
Finished Blend (S-3) |
12 hours |
|
Finished Blend (S-3) plus 0.2% Inhibitor “R” |
16 hours |
PRODUCTION DATA:
Total German production of synthetic aviation lubricating oil was as follows:
|
Design |
Actual Production |
|||
|
Metric |
B/D |
Metric Tons/Mo |
B/D |
|
|
Leuna |
850 |
206 |
700 |
170 |
|
Schkopau |
350-500 |
85-121 |
350-500 |
85-121 |
|
Pölitz |
1200 |
290 |
1000 |
242 |
|
Rhenania, Harburg |
700 |
170 |
500 |
121 |
|
Total (Max.) |
3250 |
787 |
2700 |
654 |
This production had been reduced by bombing as follows:
| Leuna - | No production after July-August 1944 because of lack of
ethane after bombing of hydrogenation plant.
In later raids the lubricating oil plant itself was damaged. The vessels and pipe line of the cracking section were badly pierced by bomb splinters but the cracked gas purification plant was not too badly damaged. The Linde refrigeration plant compressor capacity could be salvaged. The polymerization and distillation sections appeared more or less intact but storage tanks and general service piping in the area were very badly damaged. |
| Pölitz - | Production reduced to 500-600 T/Mo. in last 6 months operation. |
| Rhenania - | No production after July 1944 bombing. Plant was planned to be moved to Harz Mountains but was not completed there. |
An ultimate total production capacity of 70,000-80,000 Tons/yr (1410-1610) B/D) synthetic lubricating oil was planned and plants had been erected at Moosbierbaum and Blechhammer, but never operated.
NOTES ON CATALYSTS AND REACTION CONDITIONS
1) Acetylene Removal Catalyst
The catalyst (Kontakt 4788) is manufactured as follows:
Fifty kg. (110 lbs) chromic acid, 9.24 (20.6lbs) nickel nitrate, and 20 litres (5.3 U.S. gal) water are mixed in a stainless steel vessel. The vessel is gas-fired, and the mixture is hand-stirred for several hours until it gives a thick black mixture with no more evolution of nitrogen-gases. This mixture is heated 12 hours at 572°F in an electric-heated furnace. After cooling, the resultant hard cake is broken by hand into 3/8” (8-10 mm) granules.
The granulated mass is placed in an electrically heated reduction oven, heated in an atmosphere of nitrogen to 842°F and held at this temperature for eight hours in the presence of hydrogen. The catalyst is cooled in a stream of hydrogen and stored in containers in a nitrogen atmosphere.
The composition of the finished catalyst is approximately 95% chromic oxide and 5% nickel.
2) Ethylene Purity Required
For ethylene polymerization, the following limitations on ethylene purity must be observed:
|
Carbon dioxide |
Complete absence, no trace with barium hydroxide test |
|
Oxygen |
Complete absence, reactor must be purged with ethylene before starting operations. |
|
Hydrogen Sulphide |
Complete absence. |
|
Carbonyl Sulphide |
Complete absence |
|
Mercaptans |
Complete absence. |
|
Thiophenes |
Complete absence |
|
Carbon Monoxide |
Maximum 0.005%. Check with standard haoomoglobin solution, followed by spectroscopic determination of the width of the absorption band |
|
Hydrogen, methane, ethane, acetylene |
Total must not exceed 5%. |
3) Polymerization Catalyst
Pure alumium chloride is unsuitable as a polymerization catalyst because it is too active and permits too many side reactions, including isomerization and cracking. It is modified to a suitable form by the addition of about 4% by weight of ferric chloride. The catalyst is prepared at Ludwigshafen and Schkipau by treatment with carbon monoxide and chlorine of a natural bauxite which contains about the correct amount of iron oxide. Any traces of titanium, silicon, or magnesium chlorides present in the finished catalyst do not seem to affect its polymerizing qualities.
The maximum temperature reached during formation of the catalyst complex with ethylene in the presence of light oil controls the activity of the catalyst and the type of oil subsequently produced. The higher this maximum temperature the higher both the VI and the pour point of the product. A good compromise on these two tests calls for a maximum temperature of 356°F.
As previously mentioned the polymerization temperature govern the viscosity of the final product. According to Zorn, an oil of 6° Engler at 100°C (220 SSU at 210°F) is produced at 212-230°F and an oil of 3° Engler at 100°C (105 SSU at 210°F) at 266-284°F. These temperatures are lower than those already reported for Leuna plant operation, probably because of a difference in ethylene purity between laboratory and plant operations.
Other catalysts have been tried for ethylene polymerization properties, but without much success. Aluminium chloride plus metallic aluminium is too strong a catalyst for the reaction and hydrofluoric acid tends to form ethyl fluoride instead of a polymer.
Boron trifluoride in HF can be used as a polymerization catalyst but produces an inferior quality of oil.
THEORY OF LUBRICATION
This subject was discussed in detail with Dr. Zorn at Leuna. His work in this field has been continuous since about 1927, and he is credited with the development of the commercial synthetic lubricating oil manufacture.
Preliminary research showed that an olefine to be used for polymerization to a high V.I. lubricating oil must have a double bond at the end of a straight chain molecule, with the exception that an iso-structure at the opposide end of the molecule from the double bond may give some advantage, as discussed later. No substitution may appear at the double bond.
For instance, the following examples of isomeric octane polymerization bring out the effect of differences of structure.
|
Polymerized Product |
|||||
|
Viscosity |
|||||
|
Olefine Feed |
Mol. w. (Approx.) |
Engler |
SSU |
V.I. |
Yield |
|
n-octene-1 |
800 |
2.8 |
98 |
100 |
80 |
|
2-methylheptene-1 |
350 |
1.2 |
32 |
20 |
20 |
|
n-octene-4 |
200 |
0.8 |
28 |
-10 |
5-10 |
|
6-methylheptene-1 |
1500-1700 |
7.9 |
290 |
100 |
85-90 |
The theory based on these observation and some organic syntheses is as follows:
The ability of an oil to function as a lubricant involves a molecular structure which permits close approach of the chain carbon atoms and the metal surface so that dispersion forces between the atoms of the metal and the lubricant can become effective. For good viscosity characteristics, likewise, it is necessary that the molecules should have a structure which gives close contact of the individual molecules in order to utilize the dispersion forces active between molecules of the oil. As the magnitude of the dispersion forces increases, the viscosity index of the oil increases also, and more energy (heat) has to be added to overcome these forces and decrease the resistance to flow of the oil. This is a disadvantage insofar as the pour point of the oil increases when the viscosity index of the polymerized oil is increased. A compromise between these tow properties must be made in practice by preparing an oil with a V.I. somewhat higher than that of petroleum oils and with a reasonably low pour point.
The relation between V.I. and pour point is demonstrated by the following examples:
(1) Octene - 1 polymerized to this type of structure:
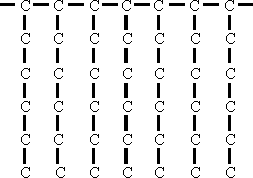 |
V.I. 110 Pour Point -40° |
This approximately the structure which is obtained by ethylene polymerization Leuna.
(2) Hexadecene - 1 polymerizes to this type of structure:
|
V.I. = 140 |
Polymerization of a branched chain olefine like 6-Methylehptene-1 yields a product of the following structure:
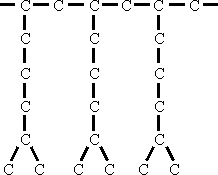
In this case the viscosity of the oil is increased because additional forces become effective between the terminal methyl groups.
Isobutylene polymerizes to give a polymer of the following structure:
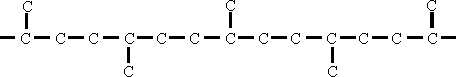
The location of carbon atoms on alternate sides of the straight chain prevents close contact between the carbon atoms in the main chain and the atoms of the metal to be lubricated. consequently the isobutylene polymer cannot be used as a lubricant, in spite of its high V.I. (110-120) and satisfactory physical properties. Bearing failure results after a few hours when this oil is used in an engine.
Similar considerations apply in the case of propylene, which apparently forms chains through the center carbon atom, thus giving side chains on both sides of the main chain. The V.I. of the polymer is about 85 and the polymer is unusually heat stable.
N-butylene with the double bond in the 1- position gives a good lubricating oil but butene -2 does not.
The change in dispersion forces between molecules is further illustrated by the change in the melting point of hexadecane upon addition of methyl groups.
| Melting Point °F | ||
| Hexadecane | +48 | |
| + 1 CH3 group | -47 | |
| + 2 CH3 groups | -126 | |
Sources of Olefines:
Olefinic material suitable for polymerization has been obtained by cracking several types of wax. The wax must be of a straight-chain structures and must be cracked in the vapour phase with no liquid present. To obtain structures with the double bond at the end of the molecule, the cracking time must be short at high temperatures, forming at least 95% olefine, followed by a quench to prevent isomerization of the olefine and migration of the double bond.
Wax from the atmospheric pressure Fischer-Tropsch process without the heaviest ends (controlled by maximum melting point of 176°F) is a suitable cracking stock. Wax from the 20-atmosphere (300 psi) Fischer-Tropsch synthesis is unsuitable because of branched chain paraffins. Wax from brown coal tar distillation is suitable for production of the required type of olefine.
Petroleum wax as such cannot be easily handled by the above process, but can be converted to suitable wax by a combination cracking-hydrogenation stop which yields a mixture of 50 per cent Diesel oil and 50 per cent straight chain paraffin wax. Hydrogenation is carried out at 3000 psi and 750°F with a tungsten sulphide catalyst and this process is in use at Ludwigshafen where 1000 tons per month slack wax from Hanover crude containing 60 per cent paraffin is processed.
The plant at Pölitz cracks a mixed wax containing Fischer-Tropsch wax, brown coal tar wax, and the special petroleum wax prepared at Ludwigshafen. A special process has been developed by Rhenania for direct utilization of petroleum wax.
Hydrocarbon Synthesis:
In the course of the study of the structure of lubricating oils, certain hydrocarbons were synthesized. The general method of synthesis is outlined as follows:
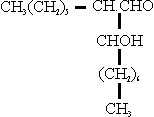
3. This product was reduced to the alcohol and converted into the iodide:
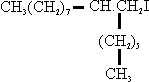
4. This compound was condensed with a suitably substituted malonic oster to give:
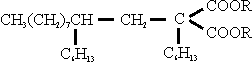
5. This product was reduced to give:
6. Steps 3 to 5 were repeated to add side chains and build compounds up to about 600 molecular weight.
LUBRICATING OIL ADDITIVES & SPECIAL PRODUCTS.
As the war progressed, it became necessary to develop lubricants, sometimes to augment dwindling supplies of otherwise satisfactory materials, but usually, as in the case of Machine Gun Oil, Recoil Oil, R.R. Axle Oil, and others, to secure improved products, usually with rust preventive properties. The development and manufacture of many of these new special materials was assigned to Dr. Zorn’s laboratory at Leuna, on account of the wide experience available there on synthetic lubricating oil.
Special attention was given to compounds which could be made readily from materials already available at Leuna and two outstanding developments were Mesulfol 2, an extreme-pressure additive, and KSE, a rust preventive. Other important blending agents of the oster type were being manufactured for inclusion in special oils used by the German armed forces. Detailed information on the blending agents made at Leuna, the methods of preparation, properties and utilization was obtained from Dr. Rössig and samples of the various products were obtained for assessment.
The individual additives are first dealt with and then the specialized lubricants incorporating these additives.
I Additives
(1) Inhibitor “R” (also known as “ZS-1”) This inhibitor does not prevent oxidation of the oil, but guides the course of oxidation so that harmful ring-sticking products are not formed. The inhibitor has no effect on pour point, bearing corrosion, wear, or lubricating properties (Schmierfähigket)
The inhibitor has the following chemical formula:
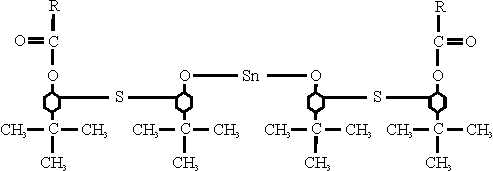
where R represents branched-chain hexyl, heptyl or octyl groups.
The steps in the synthesis of this inhibitor are as follows:
(2) E.455. A mixture of the adipic esters of 140-180°C alcohols obtained as by -products from isobutanol synthesis is prepared by heating , to 160°C, mixture of 1.6 g. alcohols, 0.48 g. adipic acid, and 0.0015 g. β-naphthalene sulphonic acid. The reaction mixture is washed with a 20% NaOH solution and distilled under 0.2 mm. Hg. pressure until the still bottom temperature reaches 100°C. The material remaining in the still is then refined with CaO and Fullers Earth.
(3) E.504. A mixture of adipic esters of 160-200°C alcohols (C8-C12) from isobutanol synthesis. Three methods of preparation were obtained:
The reaction product is washed with 20% NaOH solution and the washed ester distilled at 0.2 mm. Hg. pressure until the overheads temperature reached 110°C. The liquid remaining in the still is refinded with CaO and Fullers’ Earth.
Physical properties of the ester are:
Pour Point - 94° F.
Viscosity Index 135 - 155
Production of this ester at Leuna was about 20 tons/month. It was noted that straight chain alcohols did not give as satisfactory a product as the branched chain alcohols.
(4) E.515 Mixed adipic esters of 140-180° alcohols from isobutanol synthesis.
1.7 g. alcohol mixture, 0.42 g. adipic acid, 0.0017 g. β-naphthalene sulphonic acid are heated to 220-230°C and the reaction product washed with 20% NaOH solution. The washed ester is distilled at 0.2 mm. Hg. pressure until the overheads temperature reaches 155°C. The liquid remaining in the still is refined with Fullers’ Earth.
(5) E.3022 The ester formed between cyclochexanol and 2-methyladipic acid.
![]()
1.5 g. cyclohexanol, 0.82 g. 2-methyladipic acid and 0.002 g. β-naphthalene sulphonic acid are heated to 170°C. The reaction product is washed with caustic soda and then water. Excess cyclohexanol is distilled off and the ester distilled at 0.2 mm. Hg. The distillate is stirred with 2% CaO plus 3% Fullers’ Earth for 1 hour at 70°C to remove acid.
(6) E.3023 The adipic ester of p-methylcyclohexanol
![]()
prepared in a similar way to E.3022, from 1.5 g. p-methylcyclohexanol, 0.75 g. adipic acid and 0.002 g. β-naphthalene sulphonic acid.
(7) Mesulfol 2.
![]()
where R is C5 or C6
The particular xanthate (dissolved in ketones, alcohol or water) is reacted with ethylene dichloride at 70°C. The product is washed with water and distilled.
(8) K.S.E.
![]()
where R1=C15 radicle
R is derived from 180°-250°Calcohols
from isobutanol synthesis
0.46 g. of a C15 hydrocarbon fraction, treated with SO2 and Cl2 to give a sulphonyl chloride is reacted with 2.2 g. mesamidoacetic acid and 0.69 g. of the alcohol fraction at 150°C in the presence of 0.002 g. β-naphthalene sulphonic acid as catalyst and propyl other or ester distillate as azeotrope-forming agent to effect removal of the water produced during esterification. The reaction mixture is neutralized with carbonate solution, washed with 5% NaCl solution or distilled at 120°C (base temperature) at 10 mm. Hg., diluted with 50% middle oil and washed with a mixture of 2 parts methanol and 1 part water. the washed crude ester is distilled at 2-5 mm Hg until the still bottom temperature reaches 140°C and the liquid remaining in the still is refined with Fullers’ Earth.
Other compounds not yet used in fuels or lubricating oils, which were being studied, included
II. Special Lubricants.
(a) Waffenol Blau 44, a rust preventive gun oil containing Mesulfol 2 in an amount corresponding to 3% sulphur in the final product. The other components are ester E-455 and a light distillate from synthetic lubricating oil known as VT-120. No production figures were available.
|
Compositon: |
VT-120 |
45% |
|
E.455 |
45% |
|
|
Mesulfol 2 |
10% |
|
|
Properties: |
Viscosity |
1.9°E at 20°C |
|
Flash Point |
125°C |
|
(b) Torpedo Oil T-1 was developed as a replacement for neatsfoot oil. It was made only at Leuna and was tested at Gdynia. Total production for 1944 was 100 Tons, and the last shipment from Leuna was 40 Tons of finished product.
|
Composition: |
SS.903 |
35% |
|
E.515 |
63% |
|
|
KSE |
2% |
|
|
Properties: |
Viscosity |
11.5-12.5°E at 20°C |
|
Pour Point |
below - 50°C |
|
(c) V-weapon Oil SS-1631 was developed at Leuna after about nine months of research. A monthly production of 100-200 tons was planned, but total production amounted only 100 Tons. The last shipment of this material had been to Göttingen.
|
Composition: |
SS.903 |
25% |
|
E.515 |
72% |
|
|
KSE |
3% |
|
|
Properties: |
Viscosity |
2°E at 50°C |
|
Pour Point |
below -45°C |
|
|
Flash Point |
145°C |
|
(d) Low Pour-point oils, K.10 and K.19. were developed for use in Russia and had been reported as satisfactory in service.
|
K.10 |
K.19 |
||
|
Composition: |
SS.903 |
50% |
75% |
|
E.515 |
50% |
25% |
|
|
Properties: |
Viscosity |
165°E at 99°C |
2.0°E at 99°C |
|
Pour Point |
below -50°C |
below -45°C |
|
|
Viscosity Index |
135 |
130 |
|
(e) “Y” railroad axle oil was developed for use in extremely cold weather after the experiences of the first winter of the Russian campaign. Tests have shown that an additional 100% loading can be put on railroad cars using this lubricant and all equipment failures due to lubricants are eliminated. A test has been run using the oil as an automotive lubricant in one German army corps in Russia and all the Leuna vehicles use it. These tests showed no ring-sticking or corrosion difficulties.
|
Composition: |
“R” Oil |
80% |
|
E.504 |
20% |
|
|
Dye |
0.1% |
“R” Oil is the oil obtained on neutralizing the AlCl3 catalyst complex in synthetic lubricating oil manufacture.
|
Properties: |
Pour Point |
-45 to -50°C |
| Viscosity Index |
118-122 |
(f) Aviation hydraulic Oil was developed to pass tentative specification Do 2000, put into effect in late 1943. The oil has excellent corrosion-resistant properties and low rubber-swelling properties (less than 2% increase in volume after 24 hours immersion). A fluorescent dye was added to the oil to meet the Luftwaffe colour specification.
|
Composition |
V-120 |
84% |
|
E.3022 or E.3023 |
12% |
|
|
KSE |
4% |
|
|
Dye |
.006% |
|
|
Properties: |
Viscosity |
1.75°E at 20° C |
|
650°E at -60°Cc |
||
|
Pour Point |
-70°C |
|
|
Flash Point |
120°C |
|
Conclusions and recommendation.
The use of esters in order to obtain low pour-point lubricants merits further close study. The excellent extreme-pressure properties of Mesulfol 2 and the outstanding properties of KSE as a corrosion preventive should also be investigated further.