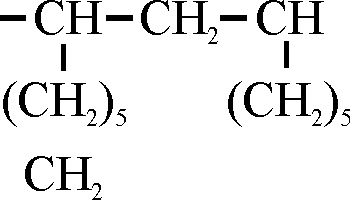
HANS SCHINDLER Ė "LUBRICANTS Ė SCHKOPAU, LEUNA AND RUHRCHEMIE"
Hans Schindler. The German synthetic lubricating oil, which was made during the prime of the war, can be divided into two different groups, one consisting of synthetic hydrocarbons and another, representing non-hydrocarbon products, mainly the esters of dibasic acids. However, this latter group, as interesting as it is, makes up only a small quantity of the entire production and was used for various specialty lubricating purposes, mainly for the Army. The bulk of the synthetic lubricating oil was bright stock to be used in blands with petroleum neutrals to make lubricating oil for the Air Force.
The two plants, Leuna and Schkopau, with which Iím concerned in this presentation, produced a total of 8,000 barrels a month of a very heavy bright stock of about 200 Saybolt seconds at 210. Two other plants were also concerned with the synthesis of lubricating oils: one at Politz near Steffin, the other at Harburg, near Hamburg, and their production brought the total synthetic oils to 20,000 barrels per month.
To anticipate the second part of my topic now, I like to mention the production of lubricating oil at Ruhrchemie; their output was of a considerably loss good grade than that made by the other plants, and corresponded to a regular motor oil of about SAE 30 grade. The Ruhrchemie production amounted to about 10,000 barrels a month.
To start out with the Leuna and schkopau procedures, it can be stated that they are identical insofar as they both make use of the polymerization of ethylene. They differ, however, in that at Leuna the theylene is prepared by a different method than at Schkopau, and that has certain consequences with respect to the ease of operation of the process. The trick in making high grade lubricating oil which the Germans found is apparently the purity of the ethylene used for polymerization, and the use of well, letís call it an especially prepared aluminum chloride catalyst. I should use those expressions rather cautiously because the catalyst is prepared, so to speak, in the process, and the aluminum chloride itself is not of special purity. The ethylene is made at Leuna by cracking of ethane which originates in the coal hydrogenation unit and is recovered from preheated to about 1200oF and mixed with about 25% of oxygen preheated to about 750oF. The reaction takes place in a special reacter consisting of a refractory-lined cylindrical vessel. Good mixing of the gases, which is important, is obtained by the use of what the Germans call the "tulip," which is nothing else than a round multi-orifice ceramic mixer. The heat of reaction raises the temperature to about 1600oC, and the entire, letís say controlled combustion of ethane is carried out under a vacuum of about 300 to 400 mm mercury, 12 percent CO, 5 percent CO2, less 1 percent of acetylene, 30 percent hydrogen, and 20 percent of unconverted ethane. The gas is cooled and then subjected to purification.
Acetylene is removed by selective hydrogenation, using a nickel chromium catalyst and a space velocity of 1,200 volumes of gas per volume of catalyst per hour, at a temperature of 428oF. The traces of oxygen which might also be in the gas are removed at the same time by conversion to water. The gas is then compressed to about 220 to 260 p.s.i., and washed with oil to remove higher boiling hydrocarbons. It is further purified by adsorption of high boiling hydrocarbons. It is further purified by adsorption of high boiling hydrocarbons on charcoal and is finally freed from CO2 by washing with Alkazid solution; youíre familiar with that. There are various formulas for the absorption solutions but the one used in this process was alpha-amine propionic acid. The gas is now substantially free from CO2 but since further separation is to be carried out in a Linde unit the last traces of CO2 must be removed and this is done by bubbling the gas through caustic solution. The gas is then dried, obviously, and fractionated in a Linde unit. Now, the purity requirements of the ethylene are such that no CO2, H2S, mercaptans, thiophene, carbon disulfide, or carbon oxysulfied must be present and the ethylene has to be at least 95 percent pure, permissible impurities being methane and not more than 1%. The CO content is limited to o.005% and was determined spectroscopically using a standard hemoglobin solution.
The manufacture of lubricating oil proper consists in the polymerization of ethylene by means of aluminum chloride, carried out as a batch process in autoclaves 40 feet high, with a diameter of about 3 feet. The autoclaves are, of course, provided with stirrers, and these are inserted from the bottom. The reactors are jacketed for temperature control by means of hot or cold water. The control of the polymerization step itself was, as I said, one of the essential features of the process and this, according to the remarks of Dr. Zorn, was successfully done by limiting the activity of the aluminum chloride in such a way that it only reacted as polymerization catalyst but not as isomerization or cracking catalyst. Therefore, a mixture of oil and 5 to 7 percent of aluminum chloride, the percentage based on the amount of finished heavy bright stock, is prepared using light oil from a previous batch, the mixture is stirred and brought to a temperature of about 248oF. At this point ethylene is admitted until a pressure of about 440 pounds per sq. in. gauge is reached and the heat of reaction raises the temperature very rapidly to about 356 degrees. Now, the addition product, or whatever it is, formed in this way, is the actual catalyst used in the polymerization. As soon as this limiting temperature of 356o is reached the cooling water is turned on, the temperature is brought down to 230o, which is the temperature level at which polymerization is carried out if heavy oil is desired, and ethylene is passed into the reactor until it is full. The pressure at the end is about 800 p.s.i. The polymerization cycle lasts about 8 to 10 hours, but rather closer to 10 hrs, since we were told that a batch could never be finished during one eight-hour shift. If for some reason a bright stock of somewhat lower viscosity, about 100 Saybolt seconds at 120 was wanted, the polymerization was carried out at 356o because at that temperature simultaneous polymerization and depolymerization takes place, which results in a product of lower viscosity than when the process is run at lower temperature. The aluminum chloride used was not of highest purity and seemed to contain about 1 percent iron. I think we will get exact figures after we have analyzed catalyst samples here. The iron concentration apparently does not seem to be critical.
The maximum temperature which is reached during the first stage, which we call the catalyst formation stage, is controlling for the V. I., and if this temperature is permitted to run higher the V. I. is higher. However, that has the accompanying disadvantage that the pour point of the oil is also raised. The autoclave product is centrifuged at about 195. F in order to remove the catalyst-oil complex. If that separation was not clean cut a small amount of methanol was added. Subsequently the centrifuged oil is freed from A1C13 and neutralized by simultaneous addition of methanol and lime. Since every polymerization reaction does not only yield the desired product but also gives products of a lesser degree of polymerization, the next step in the process consists in the removal of lower boiling material by distillation. The bright stock is finished by clay contacting. The yield of finished bright stock is about 65 percent of the total polymerization product. I donít have time to go into all the details of ethylene recovery, and so on. That information will all be found the Leuna report, which should come out very shortly.
A few words on the utilization of the A1C13-oil complex. The complex which has been centrifuged out, is again treated with methanol, the separated oil is treated with 1 percent of aluminum chloride at 158o F, and finished like the main product. A product with properties corresponding to a 100 Vis. neutral is obtained in this way, and is used for special purposes. The aluminum chloride sludge of this operation is again decomposed and yields a substitute drying oil.
Now, it is of course interesting to know a little bit about the theory behind these synthetic oils, and the Germans have done a very nice piece of work in elucidating it. It seems quite clear from synthetic studies that we have here a paraffin hydrocarbon with C6 side chains which are attached to every second hydrocarbon of the main chain with one CH2 group in between. Sounds very difficult, but if you put it on paper, itís very easily seen. (See Fig. 1) The oil which is obtained in this way has a viscosity index of at least 107 for the heavy bright stock, and 115 for the bright stock of 100 Saybolt seconds at 210, and a pour point of minus 15oF minus 31oF, respectively. The carbon residue in both cases is very low, below .2. If I might revert just a second to the theory again, it was found that if isobutylene is polymerized in the same way as ethylene, an oil is obtained which has a good high viscosity index, has all the appearances and physical inspection tests of a good lubricating oil, but when it is used in an engine the bearings are burned out after about two hours.
Fig. 1
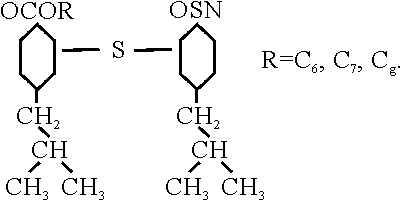
Dr. Zorn explained this observation by a lack of dispersion forces which could act on the bearing metal and this is further aggravated, so to speak, by the complete symmetry of the molecule. Something similar is found with propylene and butene-2, whereas butene-1 gives a good lubricating oil. The synthetic bright stocks which were obtained in this way were all used entirely for making aviation lubricating oil by blending were all used entirely for making aviation lubricating oil by blending with 50 percent of a good, solvent refined neutral of appropriate viscosity. The final oil comes pretty close to our aviation oil. I donít think I have to go into detail there. Interesting, however, might be the comparison of the ringsticking times which the Germans determined with the BMW 3 liter engine. The reference oil which is a Socony-Vacuum Duosol refined oil made from German crude runs 8 hours; the uninhibited aviation oil, i.e., the finished blend of synthetic bright stock and natural oil runs for 12 hours; and the same material when inhibited runs for 16 hours; the lighter viscosity bright stock alone runs for 20 hours. The inhibitor, incidentally, is a further development of a Standard Oil Company (N.J.) inhibitor. It is the tin salt of di-p-tert. Butylphenol sulfide esterified with aliphatic C6 to C8 acids which are also made Leuna. (See Fig. 2.)
The only difference between the process used in Schkopau and that in Leuna consists, as I said, in the raw material. At Schkopau, ethylene is made by hydrogenation of acetylene using a palladium catalyst and this product is purer than the ethylene made at Leuna, although apparently by ordinary analytical methods it was not possible to find differences. Spectroscopic analyses were not made. Anyway, at Leuna it was found that in order to make bright stock of high viscosity in new equipment they had to use stainless steel vessels because when they used carbon steel, which they were forced to do in the course of expansion during the war, they could for several months only make the lower, 100 Vis. Bright stock. At Schkopau, however, they only had ordinary steel vessels, but nevertheless did not have any trouble in making the high viscosity lubricating oil from the start on. That is a feature which it might be good to investigate further if any further investigations are to be carried out.
FIG. 2
Now that is very briefly the extent of manufacture of aviation stock at Leuna and Schkopau. As I mentioned before some specialty lubricants were made at Leuna, mainly esters, and these products gave some quite amazing results. For instance, axle oil for the German railroads was made by taking the oil obtained by decomposition of the A1C13-complex originating in the polymerization process and blending it with 25 to 30 percent of an ester which is made from adipic acid and a branch chained C10 alcohol. The alcohol comes from the isobutanol synthesis at Leuna. The oil which was obtained in this way has a V. I. of about 120, a pour point of minus 49 to minus 58, and a viscosity of about 100 at 100oF; it has extreme pressure characteristics and was, therefore, used as axle lubricant for German railroad cars, especially in the Russian campaign, where they had to operate at low temperatures and also were forced to overload their cars very greatly. The results were apparently satisfactory. A similar lubricant was also tried by the Germans in Russia, for automotive purposes, but apparently results never came back in any organized fashion. Leuna used the oil for their own cars and claimed good results.
Several of these esters were used for lubrication of aircraft torpedoes, for aviation hydraulic oil with rust-preventative properties, machine gun oil and lubricants for V-2 weapons and similar purposes. As I said, the output of these specialty oils was very small, it didnít amount to more than 20-30 barrels a month and this figure was not very constant, either. But in their properties these oils are rather interesting. Their main advantages are high viscosity index and low pour point.
The oil which was
made at Ruhrchemie was based on another process since in that case an olefinic
naphtha was polymerized. This material was made by cracking synthetic gas oil
from the Fischer-Tropsch process. Strictly speaking, the cracking stock consisted
of a heavy kerosene and a foots-oil which was obtained by sweating synthetic
wax. It is not quite correct as which was obtained by sweating synthetic wax.
It is not quite correct as was stated this morning that wax was used as charge
stock. What was used was actually a waxy oil, foots-oil, but not the wax itself.
Most of these products were cracked separately in a Dubbs units at 932o
to 968o F., and 74 p.s.i., using 1-2 percent by weight of steam.
The polymerization feed stock which was obtained in this way contained 70 percent
olefins. The stock, but was substantially room temperature to 390oF.,
or room temperature to 222o-198oF. The polymerization
feed stock was dried over calcium chloride to a water content not exceeding
0.015 percent. Incidentally, at this point I would like to mention that Leuna
never had any corrosion trouble with their aluminum chloride equipment because
their charge was entirely dry. The polymerization at Ruhrchemie was carried
out in reactors, but only with cooling coils. The reactor capacity was about
6,800 gallons, but only about 4,750 gallons were actually charged. About 1-1/2
percent of aluminum chloride (based on finished oil) was used as a catalyst
amounting to around 400 pounds per 4,750 gallons batch. The temperature was
very carefully controlled and slowly permitted to rise from the initial 104
degrees to a final temperature of 212oF. The total time of this batch
operation was 14 hours. The oil was next freed from catalyst which in this case
was done by settling and then dechlorinated. This was accomplished by treating
with a mixture of 1-1/2 percent of activated clay and 1-1/2 percent of zinc
oxide at 356o F for 3 hours. The material was next filtered and the
filtered cake extracted with naphtha. The filtrate still contained about .003
to .007 percent of chloride. Similar to the Leuna process, the oil also was
fractionated, the distillation at a atmospheric pressure for the removal of
gasoline and light naphtha, being followed by vacuum distillation. The finished
product in this case consisted of a neutral of about 130 viscosity and represented
about 64 percent of the autoclave charge. Abut a hundred barrels of olefinic
naphtha containing about 70 percent olefins gave about 59 barrels of finished
oil. This oil was in no way comparable to what I.G. made with respect to stability,
viscosity index, and viscosity itself. It is interesting to note that the iodine
number of this material is about 50 and the Ruhrchemie people development and
pilot plant work on an improved process, and had also designed and partially
built a plant which was, however, removed to some location in the Harz Mountains
and never put up again. This new process was capable to make a high grade viscosity
lubricating oil by using only C9 to C18 olefins for polymerization
and by adding 0.2 to 0.5 percent of phentaizine 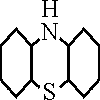 to the polymerization charge. It was claimed on the basis of experience with
the pilot plant, that this oil had a greatly improved oxidation stability, a
V. I. of around 100, and the appropriate viscosity for an aviation bright stock.
They also contemplated, by the way, to modify their feed stock preparation by
using the recycle process in which a CO-hydrogen ratio of 1 to 0.82 is obtained
by using water gas as charge stock and recycling the unconverted gas from the
first stage. But, the Ruhrchemie process, I think, is really not as interesting
as the other on in which ethylene is polymerized to a material with properties
surpassing those of natural petroleum products. As far as that goes, the oil
samples which were collected should be very carefully examined and subjected
to all the engine tests which we normally use for evaluating lubricating oils
in order to ascertain how far the quality claims which the Germans advanced
are justified. So far we are only dependent on their testimony and also their
testing methods, but as far as the carbon residue and viscosity index claims
go they certainly are correct. I think that was all.
to the polymerization charge. It was claimed on the basis of experience with
the pilot plant, that this oil had a greatly improved oxidation stability, a
V. I. of around 100, and the appropriate viscosity for an aviation bright stock.
They also contemplated, by the way, to modify their feed stock preparation by
using the recycle process in which a CO-hydrogen ratio of 1 to 0.82 is obtained
by using water gas as charge stock and recycling the unconverted gas from the
first stage. But, the Ruhrchemie process, I think, is really not as interesting
as the other on in which ethylene is polymerized to a material with properties
surpassing those of natural petroleum products. As far as that goes, the oil
samples which were collected should be very carefully examined and subjected
to all the engine tests which we normally use for evaluating lubricating oils
in order to ascertain how far the quality claims which the Germans advanced
are justified. So far we are only dependent on their testimony and also their
testing methods, but as far as the carbon residue and viscosity index claims
go they certainly are correct. I think that was all.
W. C. Schroeder. Thank you very much, Mr. Shindler, and I think I express the opinion of the group that that was a very excellent summary of the lubricating oil work. We are ten minutes behind and we still have a pretty full schedule to finish up.
J. D. Doherty. Colonel Gruen has sent Colonel Melton to discuss the matter of the foreign scientists. Heís on his way over here now.
W. C. Schroeder. Heíll be here in a few minutes.
H. M. Weir. I have two questions I would like to ask that are on my mind. I understand the polymerization of the ethylene resulted in a compound which was a long string chain of CH2 groups. Every other group had a benzene ring attached to it.
H. Schindler. No, the oil does not contain aromatic hydrocarbons, it consists strictly of paraffin hydrocarbon with no aromatics at all. I can mention that the molecular weight of the high velocity lubricating oil is around 1,000. They did experimental work with polymers of a molecular weight as high as 1,500, and, also, perhaps it is appropriate now to say that they worked out the rules very well which control the relation of structure and oil properties. It was found that in order to obtain a good yield of lubricants with high viscosity and good V.I., the double bond of the monomer had to be at the end of the chain. No other branching than the branching at the opposite end of the double bond is permissible; no branching anywhere along the chain.
H. M. Weir. Do I understand that this was a saturated ring then attached to these?
H. Schindler. No, a straight chain with the C6, so to speak, hanging down from the chain.
H. C. Atwell. At Amsterdam we were told that something like this Ruhrchemie olefin polymerization process of a mixture of olefins and aromatics had given the lubricating oil of superior oxidation resistance. Have you run into anything like that?
H. Schindler. No, we questioned the Ruhrchemie people especially on the oxidation stability and what they intended to do about it, and the only thing they were going to do was to use higher molecular weight hydrocarbons, C9 to C18, for polymerization and also incorporate phenothiazine in the original polymerization charge. Incidentally, it was interesting to find out, according to their claims, that when they added this phenothiazine to the finished oil they were never able to keep it in solution; it always precipitated out as a gel, could not be filtered out, and was a mess in general. But, if they added it before polymerization, it solved the problem quite nicely.
L. P. Evans. What information is there on propylene polymerization?
H. Shindler. Well, the only information we have is that it does not hive an oil of good lubrication properties. Butylene does, but propylene does not.
L. P. Evans. Is that isobutylene or normal butylenes?
H. Shindler. Butene-1, because they always want the double bond at the end. But propylene does not give a good lubricating oil for the same reason that isobutylene doesnít give it, because you have more or less "shielding" of the straight chain, from the next layer of the same materials or the heating metal by these little CH3 groups, whereas, if you have the long side chain apparently they act the same way as the straight chain itself.
W. C. Schroeder. Are there any further questions? If not, I think weíll move on to Dr. Hirstís presentation on "The Hydrogenation of Coal and Tar Ė Coal at Wesseling, Gelsenberg, Scholven, and Leuna; Tar at Bottrop, BŲhlen, and Zeitz."