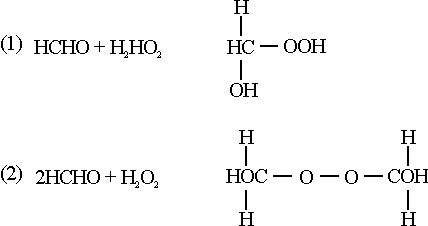
VLADIMIR HAENSEL – "SHELL RESEARCH"
Research at Shell and Hannover.
V. Haensel. Along with Shell Research, I would like to cover some of the other topics as well, particularly the research done at Hannover. The Shell research itself covers two different places, Hamburg and Amsterdam. Mr. Atwell, as I remember has gone to Amsterdam and obtained some information there. We obtained Amsterdam research reports which were sent to Hamburg and also talked with Professor Zerbe, Director of Research at Shell, Hamburg. One of the things that was of interest was the methanation reaction where they tried to react methane with various hydrocarbons.
The original research on this topic did not originate at either Amsterdam or Hamburg, but apparently attempts were made to transfer this work to Amsterdam for further study. The original idea was that methane becomes highly reactive at about 400oC and 2,000 atms. Pressure, and could be made to react with other hydrocarbons. An example of the reaction is that of pentadecane with methane to give two octane molecules. Of course, that would be a very good thing to have. Apparently, a tremendous amount of work has been done at the Concordia plant at Sterkrade, but I do not recall previous mention of this plant when we went to Ruhrchemie. They claim there is an autoclave which has a volume of 700 liters and can withstand pressures of 2,000 atms. I wonder if anyone would be interested in knowing there is such an autoclave available. Despite the tremendous amount of work they have done, there is no indication at all that methane could be reacted with hydrocarbons even under these drastic conditions. They tried all other types of hydrocarbons such as aromatic, acetylenic and olefinic hydrocarbons, but the results were absolutely zero. The idea was that Shell at Amsterdam should take over this project and start all over again, but that has not materialized.
E. B. Peck. In connection with the methanation reaction, we were told by Dr. Uhde (Dortmund) that he has been working with methane at 5,000 atms pressure and stated that at 330oC methane is very reactive, so that formaldehyde is obtained when methane is reacted with water. The work was stopped because of compressor difficulties but he was read to resume his work as soon as the equipment could be built. I talked with Dr. Lowry and one of the chemical teams going back into that area to follow up that lead to see if there is anything in the methanation reaction.
J. G. Allen. We have additional information from Professor Martin concerning the methanation reaction. I think that the first work that led to this was done at Ruhrchemie in an attempt to make organic ammonium salts followed by work by Sexauer on the use of methane as a hydrogenation agent at 500-6000c and pressures up to 3,000 atms. However, Ruhrchemie was disappointed in Sexauer since no results were obtained by him over a period of five years, therefore, the whole thing could not have been successful.
H. V. Atwell. Do we have some means of finding out what additional information CWST has on that subject?
W. G. Schroeder. We can get all the C.I.O.S. reports.
V. Haensel. There is a very complete German report in the microfilms on the methanation reaction which has photostated in Hamburg and there were a number of copies that were put into a number of bags so I am sure that some of them will get through.
The next topic, that of propane peroxide, was studied extensively at Shell Amsterdam; the valuable product from this being hydrogen peroxide. Basically, the reaction involved propane and oxygen which are brought together and allowed to react.
The work was started at the beginning of the war and the first pilot plant was constructed to operate briefly as follows: 12.7 cu.m. of 95% pure C3H8 (containing no C3H6) was mixed with 4.8 cu.m. of oxygen prior to entering a stainless steel reactor. This inlet point to the reactor was so arranged that the material would not flow along the walls but would have a spherical motion inside of the reactor. The gas mixture is fired by means of a spark plug and a temperature of 465C is maintained by varying the amount of oxygen added. Once the reaction is started, the spark plug is no longer needed, the correct temperature being maintained by regulating the amount of oxygen added. The reaction products are water, hydrogen peroxide and oxyalkyl peroxides. The oxyalkyl peroxides are formed by the interaction of formaldehyde with hydrogen peroxide:
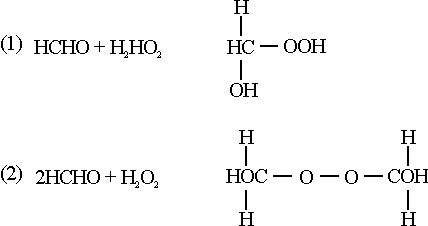
The production is cooled and is subjected to subjected to a separation operation which is finally followed by the distillation of hydrogen peroxide. The oxyalkyl peroxide is decomposed into formic acid and hydrogen peroxide and a yield is obtained, on once-through basis, of 5.2 kg. of 30% hydrogen peroxide based on 100 cu. meters of inlet propane. Of course, a considerable amount of unreacted propane is recovered as about 85% of the exit gas is unchanged propane. The propylene formed is removed because it apparently interferes with the oxidation process. The process does not operate will on air-propane mixtures, but no experiments have been made on air-propane mixtures under pressure. The Germans have tried to repeat these experiments at Leuna and, as far as I know, there was considerable doubt concerning the value of the process. If there are any comments on propane peroxide maybe we ought to have them now before I go on to the next topic.
H. Schindler. I just remembered a document where Leuna evaluated the process and said they had to use too much oxygen and too much propane to make it really an attractive proposition. So they apparently used other methods for making hydrogen peroxide but for a while they were attempting to go ahead but gave it up for these reasons.
V. Haensel. The other process at Shell Amsterdam was the so-called Mirasol extraction process. It deals with treating oils with antimony trichloride as a solvent. The main advantage of this process is that a once-through operation using this particular solvent is as good as multiple extraction with phenol or SO2 or others. The loss of antimony trichloride is said to be about one-hundredth of one per cent in a single operation. The process is carried out at the melting point on antimony trichloride, that is about 80oC and the ratio of the solvent to the solute is one to one.
A considerable amount of work was done on lube oils and one of the improvements in the preparation of lube oils by polymerization of olefinic stocks by means of aluminum chloride is the use of aluminum chloride vapor. An aluminum chloride vaporizer is located next to the vessel where the reaction takes place. The aluminum chloride vapors come over, and apparently the main advantage is that aluminum chloride is produced such finely divided form that the reaction time and the necessary amount of aluminum chloride are reduced considerably. For example, with granular aluminum chloride, 4% by weight and seven hours are needed to reach zero bromine number. With the vapor method, only 3% of aluminum chloride is required and the time is cut down to only fifteen minutes, which is considerable reduction. I do not know how far they went with this on a commercial reduction. I do not know how far they went with this on a commercial scale. The quality of the product and the yields are just about the same.
A modification of this process is the initial preparation of a paste by the vapor method using the olefin mixture or a part of the oil product as the carrier followed by contacting of fresh olefin mixture with this paste. In other words, this is a utilization of aluminum chloride sludge. The other modification is the stopping of the polymerization reaction before it is complete. The idea is to obtain different products in the beginning and in the end of the polymerization reaction. Thus, the reaction is stopped at about 60 instead of zero bromine number. The primary product is removed and unreacted material is subjected to further polymerization. Thus, a number of lube oil grades are obtained, the best one being obtained at the state and the worse at the end. A somewhat different modification is the pretreatment of the olefin mixture prior to the polymerization with aluminum chloride. Thus pretreatment of the olefin mixture with zinc chloride or clay gives lower carbon residues of the finished lube oil.
P. K. Kuhne. Dr. Haensel did not mention that this method of stopping the reaction of polymerization prior to completion, in other words at about 60 bromine number, gave lube oils having viscosity indices of the order of 140 which is higher than anything we know of in this country.
V. Haensel. That is an important point which I failed to mention. I would like to discuss now the work at Hannover which was carried out at the Technical Institute associated with the University of Hannover.
The institute was organized in 1944 and shut down in 1945, but in the meantime they worked on several interesting projects. We obtained some of the records, however, these are not complete. One of the major programs was the study of solvent extraction. Hydrazine derivatives have been found to be quite effective as well as a mixture of about 90% dioxane with 10% formic acid. A detailed report on this was microfilmed and this should be available by now.
Another topic on which they worked was the hydrogen fluoride refining of diesel oil from brown coal tar. They used 96% HF at 18oC, and the ratio of the hydrogen fluoride to the oil was 1 to 2. Contact time was about 60 minutes. The results on a typical diesel oil which was treated would be as follows: A raffinate, amounting to 59% of the charge, and an extract of 41% was obtained. This extract was actually fuel oil with 1.012 gravity and a sulfur content of 2.7%. The raffinate was divided into two parts, one was a diesel oil and the other was a lube oil, the diesel oil amounting to 32% of the original diesel oil charged. In other words, the yields were relatively low; the improvement in the refined diesel oil being an increase in cetane number from 40 to 48, decrease in sulfur content from 1.22 to 0, and a decrease in iodine number form 83 to 28.6. The lube oil had a pour point of +10 compared to –5 on the diesel oil charged. A mild steel vessel was used for this particular treatment. It appears that there is a definite polymerization reaction in addition to extraction of sulfur compounds.
The microfilm also contains data on determination of concentration of compounds containing tertiary carbon atoms, which is an analytical procedure and I believe that Mr. Baldeschweiler is familiar with that. This is about all I have on Hamburg and Hannover.
W. C. Schroeder. Well, I guess we now can move on to the next subject. There are still a lot of things to cover, which we missed yesterday. The next topic for discussion is "German Aviation Fuel Quality." George S. Bays was to have reported this, but unfortunately he is not able to beat the meetings. I am sure we will miss a good deal of the information he had prepared, but I have asked Mr. Horne to deal with the subject as well as he is able to on such relatively brief notice. Mr. W. A. Horne.