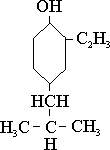
IRVIN H. JONES – "ACETYLENE CHEMISTRY (REPPE)
The New Acetylene, or Reppe, Chemistry
I. Jones. I have been delegated to discuss today with you the new acetylene chemistry otherwise known by his devotees and admirers as the Reppe chemistry. The most stimulating two consecutive days were those in which Dr. E. B. Peck and myself had opportunity to discuss this development with Dr. J. W. Reppe himself in Gendorf. To me the subject matter was entirely unknown and Dr. Reppe’s recital gave this pseudo organic chemist an atavistic thrill principally because of its implications for future developments and the startlingly fantastic structures that could be easily produced without long and laborious synthesis. Dr. Reppe can take old, tired, and sluggishly reacting compounds and insert into them an energy-rich and active group that provides them with great sensitivity and activity; for example, montan alcohol which I believe is a C29 compound, can be easily converted into a new compound that will react even with itself.
The necessity of observing in research essential but apparently minor details was pointed out by Dr. Reppe’s having noticed one little fact when studying the reaction between alkali alcoholates and vinyl halides; vinyl ethers were produced, but he also observed that simultaneously a minor amount of the acetylene was liberated in the reaction’s courts and that, after standing for some time, this acetylene in the reaction mixture decreased. This led him to the conclusion that acetylene under the influence of alkali was reacting with the alcoholate and producing more vinyl ethers. This observation let to the development of a new process for the manufacture of butadiene and also to the production of a staggering host of other reactions which even to scratch the surface of in the allotted 15 minutes would be analogous to abstracting Britannica, or Beilstein, in the same period, or in discussing the subject matter of Webster’s Dictionary in a single paragraph.
Reppe was convinced from the above observation that under proper conditions of pressure and temperature acetylene could be made to do a lot of amazing things which proved later to be the case. In order to carry out in large scale practice reactions involving large quantities of acetylene gas in pure form and also under pressure, it was necessary to know how to handle it without hazard. Its explosive characteristics were studied. He found that when it did explode the developed pressures were always ten times the partial pressure under which it was confined. There remained therefore only to build apparatus that would withstand pressures which were ten times the partial pressure under which it was used. As a further precaution all delivery pipes were filled, in honey-comb fashion, with longitudinally extended tubes of quite small diameter, thereby confining any explosion that did develop to that relatively small volume of gas within a said small pipe; also employed was continuous recirculation of the acetylene through such delivery pipes at a greater rate than it was delivered to a point of use, thereby tending further to quench the explosion. The above small pipes were in contact with each other and their metal thus tended to dissipate to the outer surface of the larger pipe in which they were contained the explosion heat and so prevent the minor explosion from expanding.
With this equipment as aid Dr. Reppe proceeded with his studies and among others he found the following interesting reactions to take place.
1. Under the influence of KOH, or the like, alcohols would react with acetylene to from vinyl ethers; similarly, also mercaptans and both aliphatic, cyclic, and aromatic amines. In this type of reaction one of the triple carbon bonds of the acetylene opens with the hydrogen of the CH, SH, or NH groups going to one of the carbon atoms and the oxy-radical, or the like, going to the other carbon atom, leaving the new compound with olefinic bond.
If zinc naphthenate, for example, is employed as the catalyst instead of KOH, and in case the alcohol is aromatic, such as phenol, the acetylene does not form an ether with the hydroxyl radical but displaces hydrogen from the phenolic ring giving vinyl phenol. One compound belonging to this class has been given the name Koresin having the following structure:
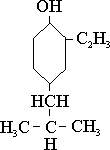
This material is extensively employed after some polymerization in the Buna industry as an adhesive in tire manufacture and was a great boon to Germany during the war.
2. Another class of reactions was that of acetylene with aldehydes. In this reaction the acetylene added to the aldehyde chain to give longer chain carbon compound, the formed hydroxyl radical appearing in terminal position, for example.
CH20 + C2H2-à propargyl alcohol,
a most interesting compound which still contains the acetylene linkage, and is in reality acetylene methyl alcohol. This compound has a number of interesting reactions. It can be hydrogenated in neutral or alkaline solution to yield allyl or N-propyl alcohol; in acid solution the propargyl alcohol is converted almost quantitively to propionaldehyde.
The hydrogen atom on the acetylene residue of propargyl alcohol can be oxidized away with cupric chloride to give a six-carbon atom di-alcohol—hexadiindiol; 2, 4-diin-1, 6-diol.
When both of the hydrogen atoms on acetylene are reacted with, for example, the above formaldehyde, we arrive at butin 1,4,-diol, a compound containing in the plane of symmetry the acetylene linkage. This compound can be easily hydrogenated to butenediol and butanediol 1,4; by removal of 2 moles of water from the latter, butadiene is produced. By removal of only one mole of water from said butanediol, tetrahydrofuran is produced which Dr. Reppe said will have a great technical future because it is an almost universal solvent and from his description of materials soluble therein, this is believable and there seemed to be excluded only man’s conscience which we know is soluble in another oxygen-containing compound.
Reaction of tetrahydrofuran with ammonia forms pyrollidine.
A substitute blood plasma which used in the German army during the war is polyvinyl pyrollidone. This is formed by the following reactions: Butanediol 1,4 is oxidized sufficiently to produce aldehyde from both hydroxy groups; the Cannizaro reaction automatically produces hydroxy butyric acid which then dehydrates to give butyrolactone. Reaction of the latter with ammonia gives pyrollidone and vinylation of its nitrogen atom with the acetylene results in vinyl pyrollidone. Subsequent polymerization of the latter with H202, or the like, gives polyvinyl pyrollidone, substitute blood plasma which has the stated advantage that is "Abgebaut" in the body and is eliminated therefrom.
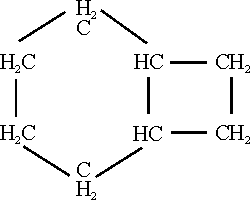
3. Cyclopolyolefins. Dr. Reppe discussed his recent developments in the field of cyclopolyolefins, the most important of which he had produced is cyclooctateraene which has the empirical formula C8H8 and is a cyclic compound, which is the counterpart of C6H6, benzene, in the aromatic series of compounds. It has four conjugated double bonds. Dr. Reppe showed us at least a liter of this interesting material which was slightly aromatic in odor and which was relatively easily produced as later described; Dr. Willstaetter had produced about 1/5 of a gram of this material from about 5 kilos of raw material by a long series of tedious syntheses.
In the preparation of cyclopolyolefins acetylene at a pressure of 10-20 atmospheres using nitrogen as a diluent and such neutral solvent as especially tetrahydofuran is converted at temperatures of 60-70oC and up to 130-140oC in the presence of such nickel compounds as its cyanide, thiocyanate, or halide into cyclopolyolefins by condensation with itself. The yield of product from acetylene is about 90%. The C8H8 component predominates along with minor amounts of C10H10 and C12H12.
In consequence of its olefinic character cyclooctateraene is very reactive in the presence of various reagents:
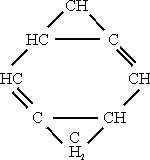
In certain of its reactions, cyclooctateraene:
Bicyclo – [0.2.4.] – octane, m.p. 1360oC.
I think, gentlemen, my time is finished, and I recommend to you the Peck-Jones report (T.O.M. Report No. 12). It’s very interesting reading.
W. C. Schroeder. Did you finish the entire report in your discussion?
I. H. Jones. No, not all the details are there; also I didn’t take up the carbonylation reactions at all; which are interesting processes.
W. C. Schroeder. Well, because you have that in such a nicely written form, not only from the standpoint of technology, but extremely interesting to hear, why can’t we just reproduce the whole thing from your written copy.
I. H. Jones. There are just notes.
W. C. Schroeder. I thought you were reading.
I. H. Jones. I abstracted the original last night in these little notes I was using.
W. C. Schroeder. Is that report a TAC report?
I. H. Jones. Yes, it’s already in. (TAC SnMC-6).
W. C. Schroeder. Well, then, we’ll use it just the way you gave it on the record.
V. Haensel. I’d like to find out if there is any other mention of zinc naphthenate. There is a whole number of naphthenate acids; has he stated anything as to which particular naphthenate acids were used?
I. H. Jones. No, in the course of the discussion, he merely mentioned this particular compound. Do you check that Dr. Peck?
E. B. Peck. I’d like to make one remark on this thing. Of course, we talked to Reppe two days and he had no notes, and lots of things we had to ask he couldn’t answer; but he said we could get at the documents. Well, we were short of transportation; in fact, we didn’t have any transportation. We went into Gendorf on the good offices of Colonel Philip Tarr of the Chemical Warfare Group and had to leave when they said so, but Dr. Ambrose promised us, on all that’s holy, that we would have all the Reppe documents, and we’d have them at a very definite time. They were out in a freight car where they couldn’t easily get them and, as far as we know Ambrose made good on that, because a CAFT operator went down there on the day after we left and got this large mass of documents and they were brought to the T-Force headquarters in Munich, as they should have been. A lot of people coming through New York called me and accused me of stealing the Reppe documents because nobody found them, and the story I got here at this meeting from somebody was that the T-Force moved from one place to another and as far as anyone knows, those documents never got past Munich. I guess, as Dr. Faragher told me, that Dr. Ambrose is just as much disturbed about the loss of those documents as we are, because they represent the only copies of any of Reppe’s work. Now that’s right in the hands of the Army and these valuable documents give just the details that we all want to get, and the I.G. people acted in good faith in giving them to us, as far as we know.
I. H. Jones. I might say that we had two or three (Mr. Paul Jones, Major Scott, and Major Hawkinson make individual trips from London onto the continent to locate those documents. They apparently were out at that town near Munich (just about ten miles from Munich) which was the T-Force headquarters last May, and they were there a time and were finally taken into Munich itself and deposited in the Haus der Deutschen Kunst and then they were taken to SHAEF headquarters in Versailles. The bags began to come apart and be broken at that time and eventually they were transferred from Versailles to Frankfurt when SHAEF moved to the latter place. If they reached Frankfurt, they may be either found in Greisheim, or it is thought they might turn up in the Heidelburg library.
W. C. Schroeder. Well, if there’s a chance of them turning up in the Heidelburg library perhaps Mr. Von Elbe can locate them and see what he can do about them.
I. H. Jones. It is felt that they are not lost.
W. C. Schroeder. I don’t want, however, to get into an involved discussion on these papers until this afternoon because it’s going to eat up too much time. We may have some time left this afternoon, and if there are not more questions bearing directly on the technology of the Acetylene Chemistry, I’m going to move onto the next paper. The next paper is "Solvent Extraction," by Mr. J.P. Jones.