Return to Table of Contents
Fluidized Bed Work at a Fuel Research Board
Work on the fluidized synthesis process has been done in an internally
baffled reactor having an internal diameter of 1” and a length of 10 feet.
The unit operates at 20 atmospheres, 300°C.,
linear velocity (based on empty tube) 0.6 ft./sec. Work has been done on an impregnated mill scale catalyst and
on a synthetic ammonia catalyst. The
fresh gas SVH was 1000 based on the volume of the volume of the settled bed.
All work was done with 2H2:1CO gas.
The following results were obtained in some of the early runs to
determine the effect of scrubbing the recycle gas:
|
|
Synthetic Ammonia Catalyst |
Mill Scale Catalyst |
|
|
End gas
Stripped
|
End gas
unstripped
|
End gas
stripped
|
End gas
unstripped
|
|
CH4, g./m.3
fresh feed
|
44
|
55
|
25
|
33
|
|
C2-C4, g./m.3
fresh feed
|
100
|
60
|
80
|
60
|
|
C5+, g./m.3
fresh feed
|
41
|
65
|
65
|
90
|
|
Total
|
185
|
180
|
170
|
183
|
|
Water sol. Oxygenated
|
2
|
5
|
14
|
10
|
|
CO going to C, %
|
1.3
|
1.9
|
1.8
|
3.0
|
The increase in the C5+ fraction recycling unstripped gas
indicates that propylene and butylenes are entering into the reaction to form
higher hydrocarbons. The increased
methane yield under these conditions is an indication of cracking of some of the
recycled C2-C4 fraction.
One experiment was begun with 400 cc. Of unreduced synthetic NH3
catalyst weighing 1 Kg. and containing 720 g. of iron.
During a 40 day run, 10000 cc. Of solids (catalyst + carbon + adsorbed
wax) having an average carbon content of 65% by weight were withdrawn from the
system. Throughout this period the
CO conversion was 90% or greater in spite of the fact that the iron remaining in
the reactor was only about 5% of its initial value.
To obtain further information on the synthesis over used catalysts
containing low concentrations of iron, the following comparison was made between
fresh mill scale and a mill scale catalyst that had been used in the synthesis:
|
Catalyst
|
Fresh Mill
Scale
|
Mill Scale
Catalyst containing carbon deposited in synthesis1/
|
|
Duration, days
|
7
|
12
|
|
Initial volume of catalyst, cc.
|
380
|
400
|
|
Final volume of catalyst, cc.
|
1683
|
1354
|
|
Fe, grams
|
720
|
70
|
|
Temperature, °C.
|
300
|
340
|
|
Initial SVH
|
1040
|
1030
|
|
Final SVH
|
743
|
317
|
|
Liters of synthesis gas/g. Fe/hour
|
0.57
|
5.9
|
|
Yield stabilized C5+ g./m.3
fresh feed
|
74.2
|
90
|
|
1/ Carbon 50% by weight; bulk density 0.42
g./ml.
|
It will be observed that the initial volume of catalyst,
initial space velocity, and temperature were the same with both fresh and used
catalyst but the iron present in the reactor containing used catalyst was only
10% of that with the fresh catalyst. Consequently,
the rate of synthesis gas feed per gram of iron with the used catalyst was 10
times that with the fresh catalyst. In
spite of this the yield of liquids plus solids was greater with the used
catalyst of low iron content.
Hall has on his program the determination of the minimum content of iron
necessary for the synthesis in a fluidized bed.
The details of a run (Run 13) with mill scale catalyst is a fluid bed are
as follows:
|
Pressure
|
20 atmospheres
|
|
Temperature
|
300-320°C.
|
|
H2:CO
|
2:1 in fresh feed
|
|
H2:CO, usage ratio
|
1.88:1.9
|
|
Velocity of gas
|
0.65 ft./sec. (based on empty tube)
|
|
Recycle ratio
|
5:1
|
|
CO converted
|
98.5%
|
|
CO à
CO2
|
4.5%
|
|
CO à
CH4
|
14.3%
|
|
CO à
C
|
1.6%
|
|
CO à
C5+
|
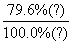
|
|
CH4, g./m.3 fresh feed
|
32-38
|
|
C3+C4, g./m.3
fresh feed
|
63
|
|
Liquids + solids, g./m.3 fresh feed
|
90
|
|
C2
|
?
|
|
Distillation of liquids + solids:
|
|
below 150°C.
|
64% by weight
|
|
below 200°
C.
|
76.6% by weight
|
|
below 300°C.
|
92.5% by weight
|
|
residue
|
5.8% by weight
|
|
loss
|
1.7% by weight
|
|
Alcohols in water layer
|
5.3-9 g./m.3 fresh feed
|
|
Fatty acids(calculated as acetic)
|
2.0-3.8 g./m.3 fresh feed
|
The minimum CH4 that Hall has observed in fluidized operation
is 25 g./m.3 of fresh feed.
The synthetic ammonia catalyst gives less oxygenated compounds than the
mill scale catalyst.
Hall’s result with the three processes and with a synthetic ammonia
catalyst have indicated that carbon formation in grams c/gram Fe/hour is
independent of the type of process in the range 0-20 atmospheres.
These results have been shown on a curve having the characteristics shown
in figure 1. The formation of
carbon is not critical until temperatures in the vicinity of 300°C. are reached. Less
active catalysts tend to form less carbon.
Return to Table of Contents